
The Traffic Light System (TLS) is a colour-coded framework designed to provide clear guidance on prescribing responsibilities and commissioning intentions for a range of selected products. It aims to clarify the division of clinical and prescribing responsibilities between specialists and primary care practitioners when a product is prescribed. This system helps ensure that prescribing decisions are made in the most appropriate clinical context, with clear delineation of who holds responsibility for prescribing.
The TLS has been developed in collaboration with clinicians from Doncaster & Bassetlaw, meaning that its guidelines may differ from those established in other regions or by external bodies. The decision-making process for including medicines in the system, or for reclassifying them within the various TLS categories, takes into account several key factors:
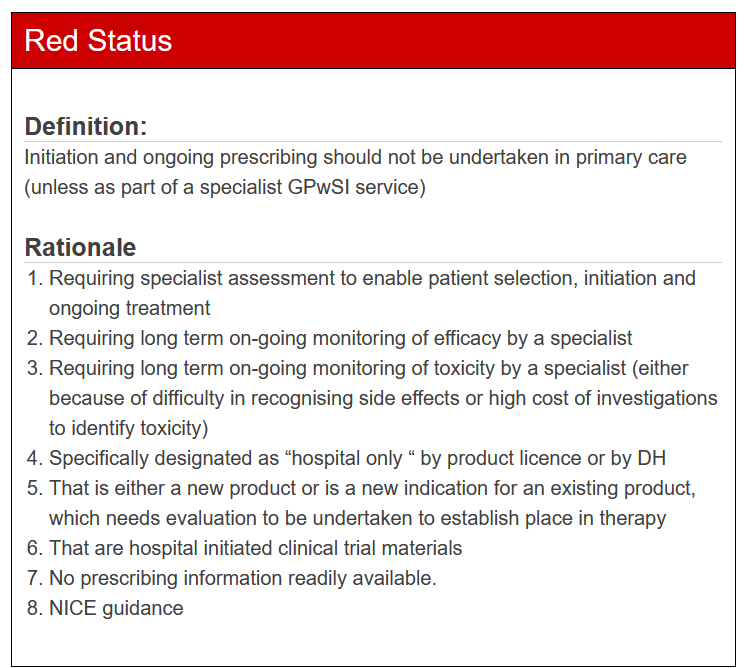
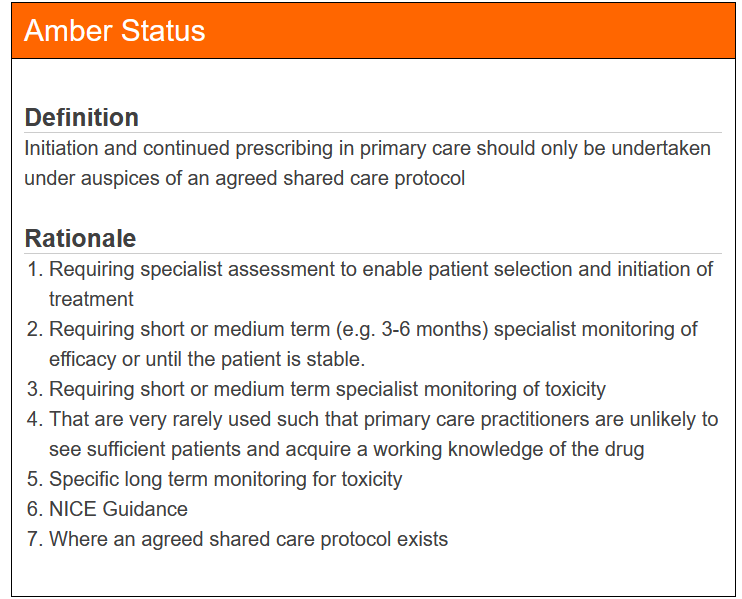
Medicines are classified into one of the following categories:
The classification of each product determines under what conditions it should be prescribed, whether it should be prescribed at all, and any specific guidance or rationale that must be taken into account. The system is regularly reviewed, particularly when new information or guidance about a product becomes available, ensuring that the TLS remains up-to-date and reflects the best clinical practice.
Request for a new TLS or a revision of an existing TLS can be made via the form below:




Welcome to Doncaster LMC. We use cookies to enhance your experience, analyse site traffic, and personalise content. By clicking 'Accept,' you consent to our use of cookies. You can manage your cookie preferences or withdraw consent at any time by adjusting your settings. For more details, please review our Cookie Policy Learn more